autoclave performance qualification|htm guidelines for autoclave : solutions 18. 24-12-2020 18 The operational qualification process is intended to demonstrate that the components are operating properly and ready for performance or load testing. Operational qualification shall be done “without load” "Operational qualification (OQ) is the process of demonstrating that an instrument will function according to its operational specification in the .
WEBPeople Playground YouTube channel (Studio Minus)
{plog:ftitle_list}
WEB17 de jul. de 2017 · According to CNN, the 16-year-old girl, who is being identified only as “Nadia” in an effort to keep her name private, drew a photo of a girl with the name “Rina Palenkova” written underneath.


The autoclave is qualified by conducting for at least Three Consecutive Separate Successful Runs to Ensure that the results are consistent and meaningful. Qualification cycle as per below .2 of 39 Autoclaves: Qualification & Validation Holger Fabritz - Expertentreff 14. September 2007 in Baden Contents • Types of autoclaves • Regulatory Aspects • GMP Risk Analysis • URS / FDS • Design Qualification • Installation Qualification / Operational Qualification • Performance Qualification / Process Validation • Responsibilities • Summary
usp guidelines for autoclave validation
Performance Qualification is an essential part of the qualification mechanism. The scope of this protocol Cum-Report is to provide complete documentary evidence for the performance qualification of the Autoclave. The protocol Cum-Reports cover all aspects of Performance Qualification for Autoclave Serving XYZ Ltd.,It is generally accepted that terminally sterilized injectable articles or critical devices purporting to be sterile, when processed in the autoclave, attain a 10 –6 microbial survivor probability, i.e., assurance of less than 1 chance in 1 million that viable microorganisms are present in the sterilized article or dosage form. With heat-stable articles, the approach often is to considerably .Keywords: Autoclave Qualification, Performance Evaluation Test INTRODUCTION As we know autoclave plays a curtail role in sterilization within the pharmaceutical and medical industries. For sterilizing materials, autoclaves are widely used. It can sterilize solids, liquids, hollows, and instruments of various shapes and sizes.18. 24-12-2020 18 The operational qualification process is intended to demonstrate that the components are operating properly and ready for performance or load testing. Operational qualification shall be done “without load” "Operational qualification (OQ) is the process of demonstrating that an instrument will function according to its operational specification in the .
Autoclave Performance Qualification Protocol, Purpose. Autoclave Performance Qualification Protocol, To authenticate and document that the performance of the Autoclave (Steam sterilizer) of Sterile Production area (Cephalosporin Block) of XX Pharmaceutical Limited (LPL) is satisfactory in all critical aspects related to the operational requirements during .
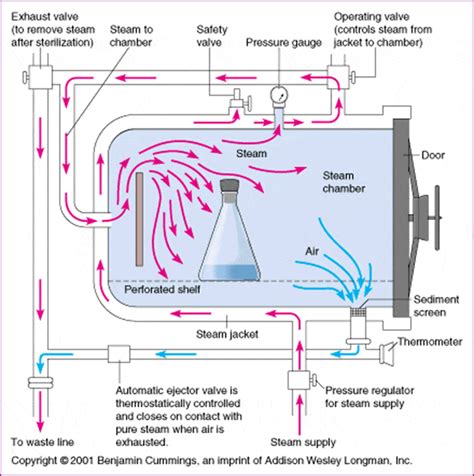
PQ - Performance Qualification Performance Qualification is what determines how effective the sterilizer is to sterilize the loads specified in the customers URS. In production manufacturing terms it is typically used to establish if the sterilizer performs to the same parameters set in OQ but with the loads added.Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Installation Qualification (IQ): Ensuring Correct Installation This phase verifies that the autoclave is installed correctly and that all required components, utilities, and equipment are present and meet design specifications. The process of confirming the autoclave is operating within the designated parameters is called the Performance Qualification. The Performance Qualification process is detailed in Australian Standard AS/NZS: 4815:2006 Office-based health care facilities – Reprocessing of useable medical and surgical instruments and equipment, and maintenance .Autoclave cycles for liquids generally heat up and cool down without a vacuum. Steam, introduced into the top of the chamber, displaces the air. The air is pushed to the bottom of the chamber and is removed. PharmOut white paper: Top .
(psi=pounds per square inch). After loading and starting the autoclave, the processing time is measured after the autoclave reaches normal operating conditions of 121 °C (250°F) and 15 psi pressure, NOT simply from the time we push the “on” button. The different tests are follows for qualification of autoclave are: – Vacuum leak test 6 11 Moist Heat • not suitable for non-aqueous/dry preparations • preferred method of sterilization 12 Dry Heat • Lethality due to oxidative processes • Higher temperatures and longer exposure times required • Typical cycles: – 160°C for 120 minutes – 170°C for 60 minutes – 180°C for 30 minutes – tunnels used for the sterilisation of glass vials may use much higher .
PQ- Performance Qualification. Once the autoclave has been proven to function without a product, the performance of the device is checked according to the specifications of the validation plan defined in advance, with the aim of proving that the specified process requirements are adequately met under real conditions (with a product). It should . Performance Qualification Protocol for Steam Sterilizer (Autoclave) and Procedure for Vacuum Leak Test, Steam Quality Test, Bowie-Dick Test, Heat Distribution Test, Hest Penetration Test and F0 Calculation. Validation of the autoclave as a prerequisite for correct application. When validating the autoclave, it is elementary that not only all applicable legal regulations are complied with, but also thatall necessary steps .
Astell UK is the leading autoclave manufacturer, offering a range of Sterilizers and Autoclaves from 33-2,000 Litres. Skip to main content +44 (0)20 8309 2031. Home; Products; . Autoclave validation and Qualification. Calibration and . Cycle development is an important part of both the autoclave procurement and validation processes. In previous posts about validation, we explored Installation Qualification (IQ), Operation Qualification (OQ), and . Using the sampling tables, select a number of training and qualification records for process operators and employees conducting Q.C. activities related to the sterilization process.The qualification of an autoclave consists of an installation qualification (IQ) and operational qualification (OQ): Installation Qualification: This verifies that your system was installed properly – and is typically performed by the system provider; Operational Qualification: This tests whether your system works as intended or not.
There is an array of qualification tests that can be conducted to validate an autoclave. Many laboratories validate autoclaves by simply using biological indicators (BI’s). For some labs, however, a simple validation with BI’s is not enough and a more elaborate validation process must be followed.PDF | On Jun 1, 2022, Faiz Nasrullah published Protokol Kualifikasi Kinerja (Performance Qualification) -Steam/Air Cycle (Autoclave) | Find, read and cite all the research you need on ResearchGate This Educational Session will provide an overview of microbiology principles in steam sterilization and application, as well as autoclave Performance Qualifi.All parts of autoclave used for measurement like temperature sensors, pressure gauges, and timers must be calibrated. 10.2 TEST EQUIPMENT CALIBRATION: . PERFORMANCE QUALIFICATION PROTOCOL CUM REPORT FOR HIGH PRESSURE HIGH VACUUM STEAM STERILIZER PHARMA DEVILS
Performance qualification is documented evidence to prove that equipment/system is performing under specified condition. It involve in taking trial under “loaded condition”. The different tests are follows for qualification of autoclave are. Vacuum leak test
Services include installation, operation, and performance qualification (IQ/OQ/PQ) protocols and/or execution of these protocols. These component programs, explained below, may be purchased individually or as a complete suite of professional services.Performance qualification of an autoclave is an involved process including worst-case assessment, cycle development and includes temperature mapping with validated data-logging probes. We only found one firm in town that was able to quote on a contract PQ program, a quote nearly 2x the cost of the autoclave of course. .
Performance Qualification Protocol (PQP) – Steam/Air Cycle- Heat distribution. Performance Qualification Protocol (PQP) for Steam/Air Cycle in the Production Steam Steriliser (Autoclave) The Production Steam Steriliser (autoclave), shall be used for sterilising aseptically-filled vials of selected products.
pda guidelines for autoclave validation

function of analyzer in polarimeter
htm guidelines for autoclave
11 min Abigail666Ja -. 720p. PEGUEI NAMORADO BATEND.
autoclave performance qualification|htm guidelines for autoclave